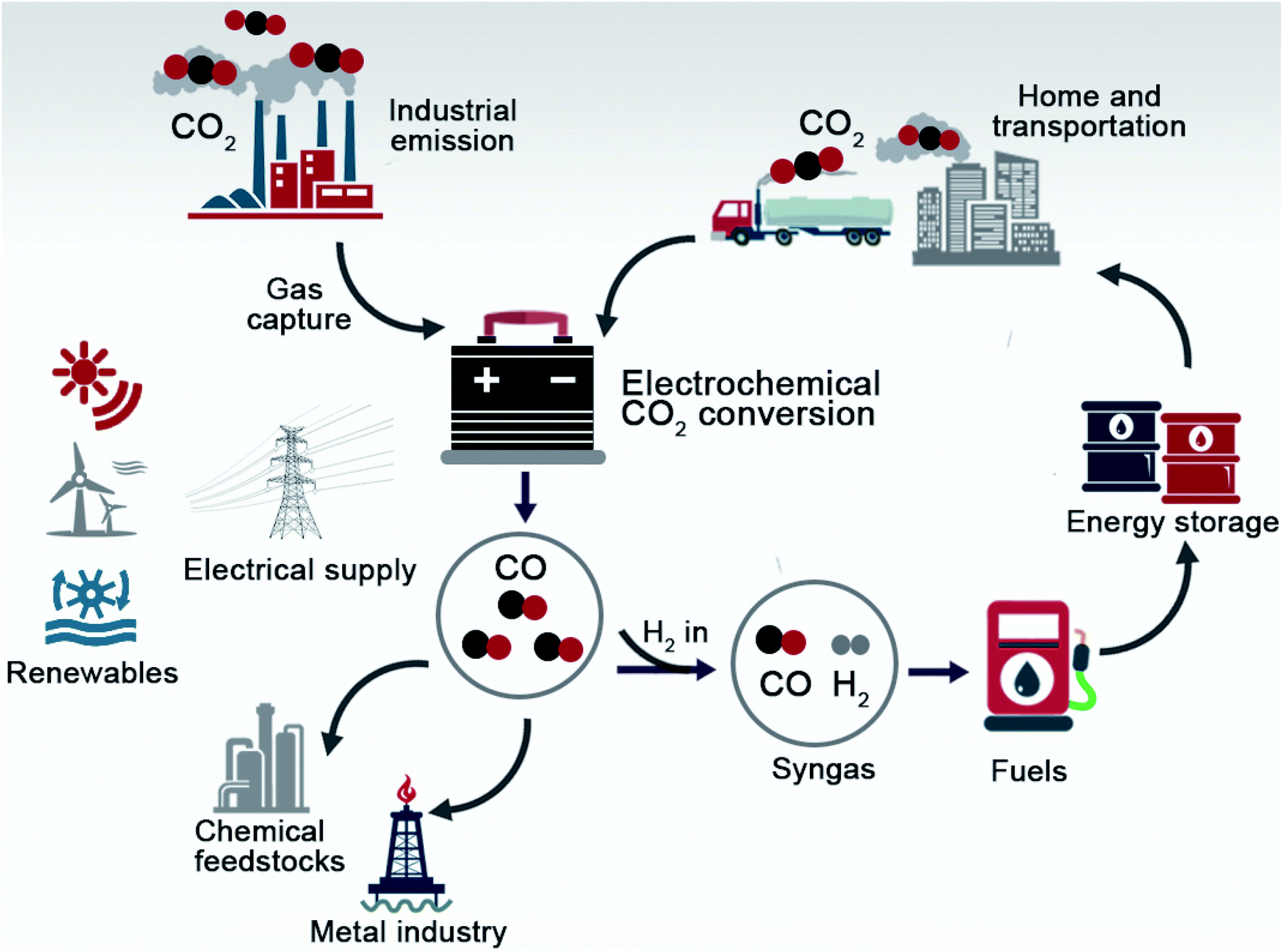
Electrochemical CO 2 -to-CO conversion: electrocatalysts, electrolytes, and electrolyzers - Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/D0TA03525D

CO2-Promoted Reactions: An Emerging Concept for the Synthesis of Fine Chemicals and Pharmaceuticals | ACS Catalysis
Is there any calculator for calculating the volume change in carbon dioxide(gas) from a thermal expansion? - Quora

Urea derivatives from carbon dioxide and amines by guanidine catalysis: Easy access to imidazolidin-2-ones under solvent-free conditions - ScienceDirect

Calculate the volume occupied by 8.8 g of CO2 at 31.1^0C and 1 bar pressure. R = 0.083 bar litre K^-1 mole^-1 .

Environmentally Friendly, Co-catalyst-Free Chemical Fixation of CO2 at Mild Conditions Using Dual-Walled Nitrogen-Rich Three-Dimensional Porous Metal–Organic Frameworks | Inorganic Chemistry

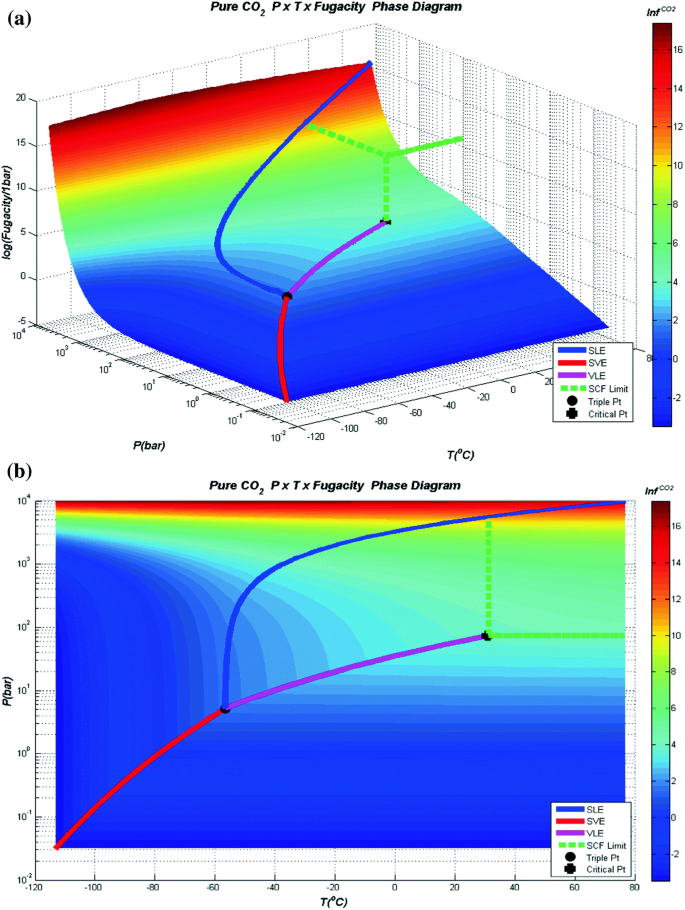
An Accurate and Efficient Look-up Table Equation of State for Two-Phase Compressible Flow Simulations of Carbon Dioxide | Industrial & Engineering Chemistry Research

Metal–CO2 Electrochemistry: From CO2 Recycling to Energy Storage - Wang - 2021 - Advanced Energy Materials - Wiley Online Library

Supercritical carbon dioxide technology in synthesis, modification, and recycling of battery materials - Han - Carbon Neutralization - Wiley Online Library

An Accurate and Efficient Look-up Table Equation of State for Two-Phase Compressible Flow Simulations of Carbon Dioxide | Industrial & Engineering Chemistry Research

Effects of Particle Diameter and Inlet Flow Rate on Gas–Solid Flow Patterns of Fluidized Bed | ACS Omega
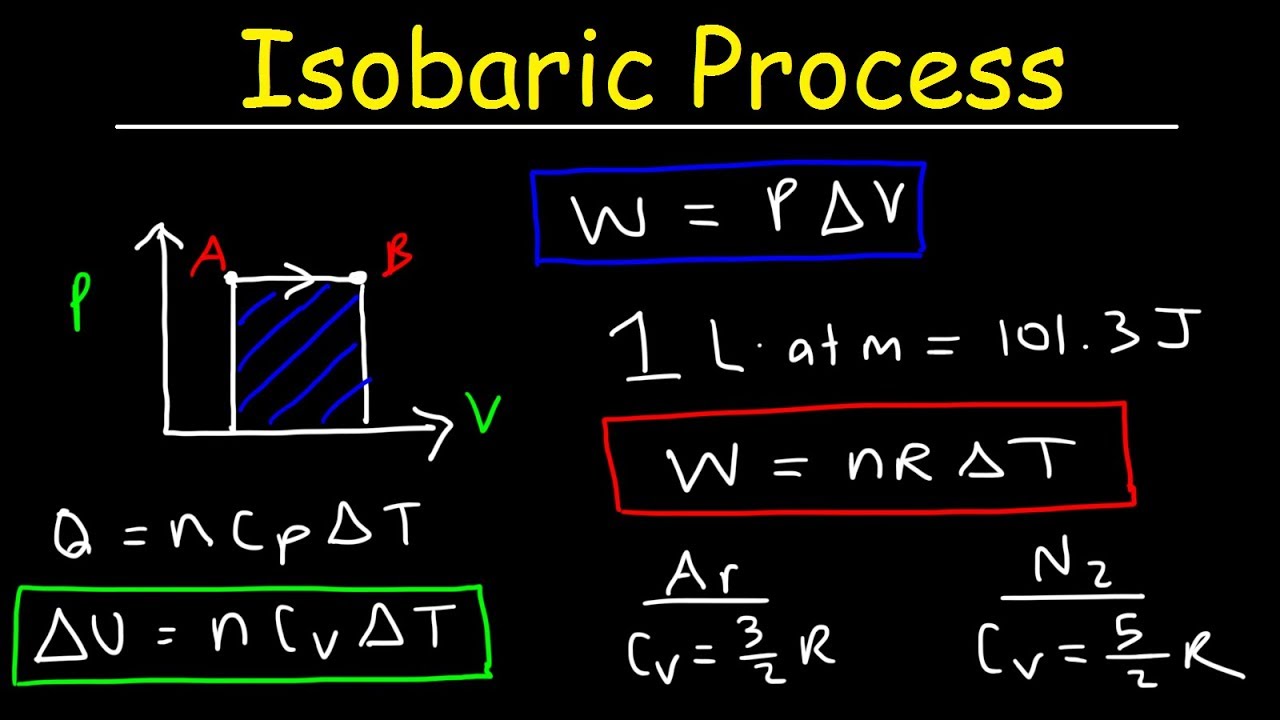
Isobaric Process Thermodynamics - Work & Heat Energy, Molar Heat Capacity, & Internal Energy - YouTube

22 g of CO2 at 27^0 C is mixed in a closed container with 16 g of O2 at 37^0 C. It both gases are considered as ideal kinetic theory gases, then

Mechanically Constrained Catalytic Mn(CO)3Br Single Sites in a Two-Dimensional Covalent Organic Framework for CO2 Electroreduction in H2O | ACS Catalysis

Environmentally Friendly, Co-catalyst-Free Chemical Fixation of CO2 at Mild Conditions Using Dual-Walled Nitrogen-Rich Three-Dimensional Porous Metal–Organic Frameworks | Inorganic Chemistry
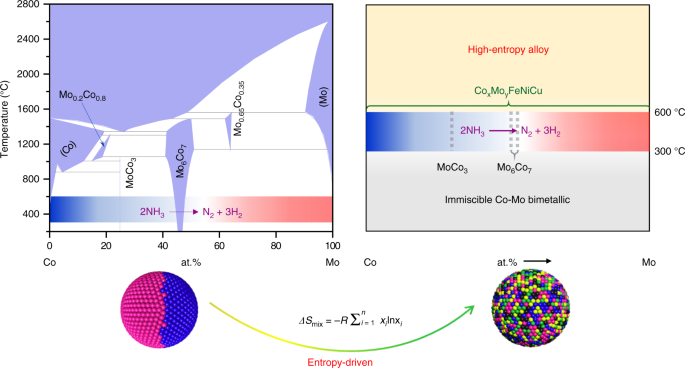
Highly efficient decomposition of ammonia using high-entropy alloy catalysts | Nature Communications