
Question Video: Calculating the Concentration of H⁺ Ions Given the Acid Dissociation Constant and Degree of Dissociation | Nagwa

the dissociation constant of a weak acid h a is 1 2 into 10 to the power minus 10 calculate - Chemistry - Equilibrium - 13209509 | Meritnation.com

The dissociation constant of a week monobasic acid is 3.5 × 10^-8 . calculate its degree of dissociation in 0.05 M solution. ( 8.37 × 10^-4 )
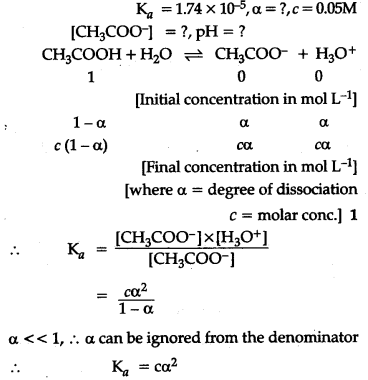
The ionization constant of acetic add is 1.74 x${{10}^{-5}}$ . Calculate the degree of dissociation of acetic add in its 0.05 M solution. Calculate the concentration of acetate in the solution and
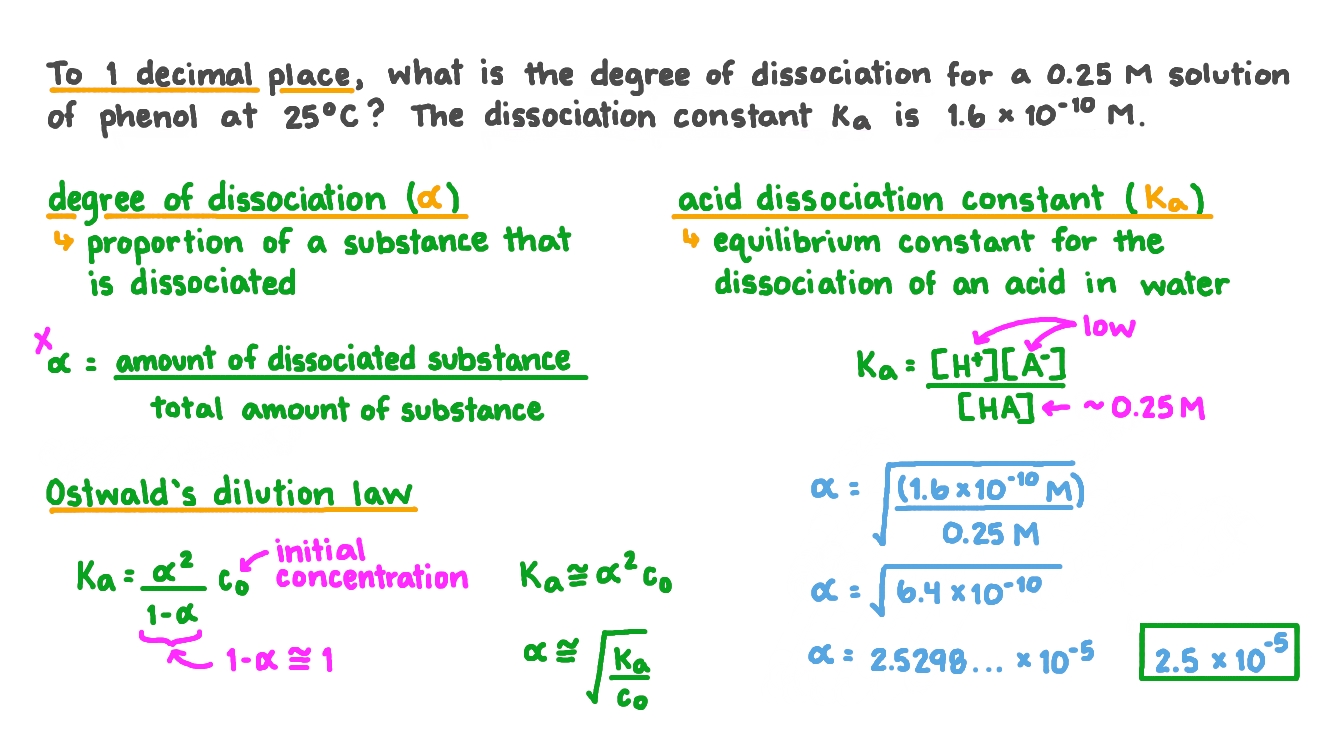
Question Video: Calculating the Degree of Dissociation of a Solution of Phenol Given the Acid Dissociation Constant | Nagwa

A certain weak acid has dissociation constant of 1.0 × 10^-4 . The equilibrium constant for its reaction with a strong base is:

equilibrium - How to calculate the dissociation constant of a weak acid from the titration with a strong base? - Chemistry Stack Exchange






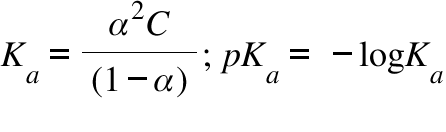
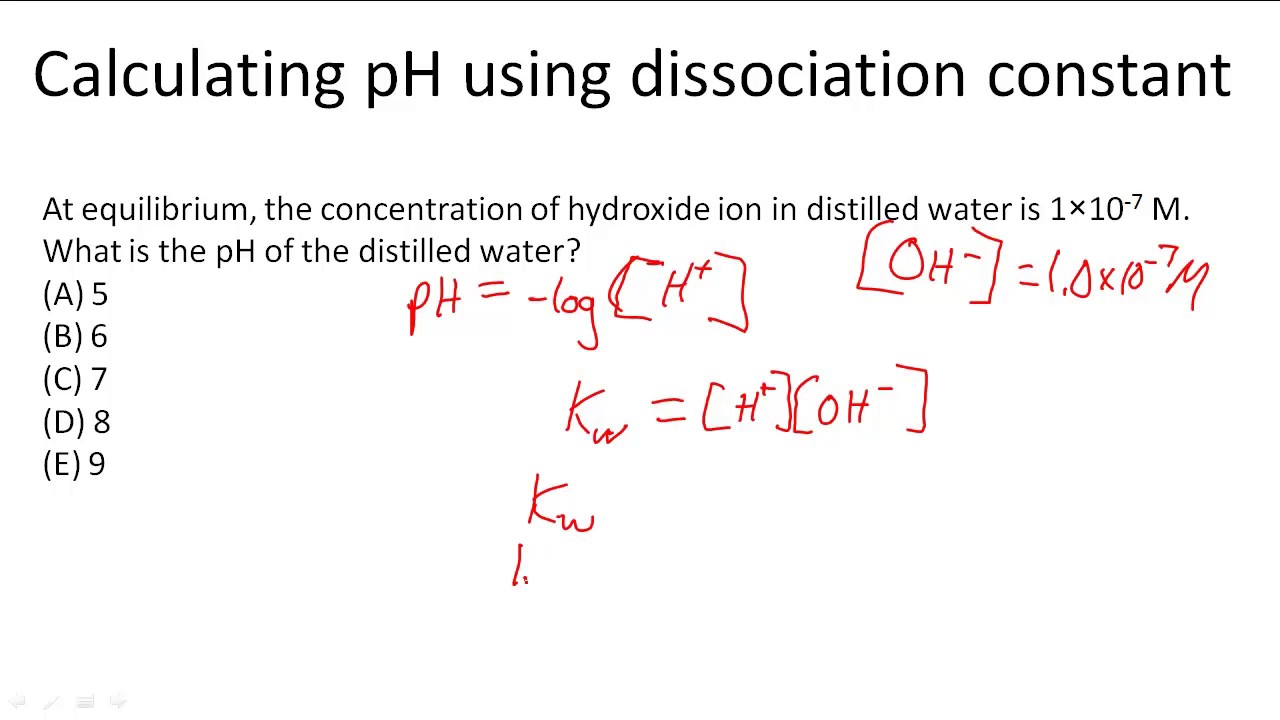


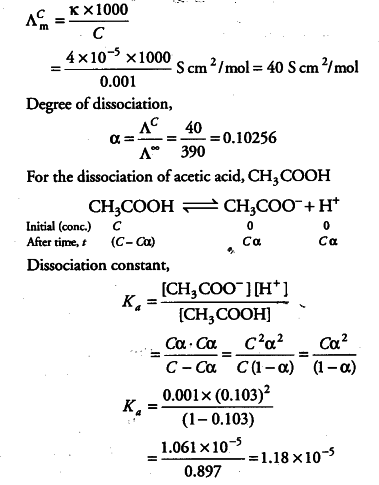


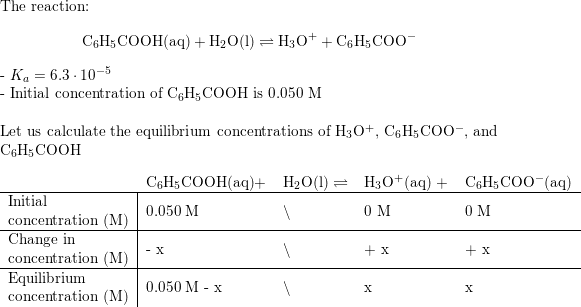
:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)

