![The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube](https://i.ytimg.com/vi/KVj56QvOV1I/maxresdefault.jpg)
The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube

What is magnetic moment and how can we find magnetic moment of Fe3+!! Pls someone help me !! - Chemistry - - 15334915 | Meritnation.com
![The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/1263733.jpg)
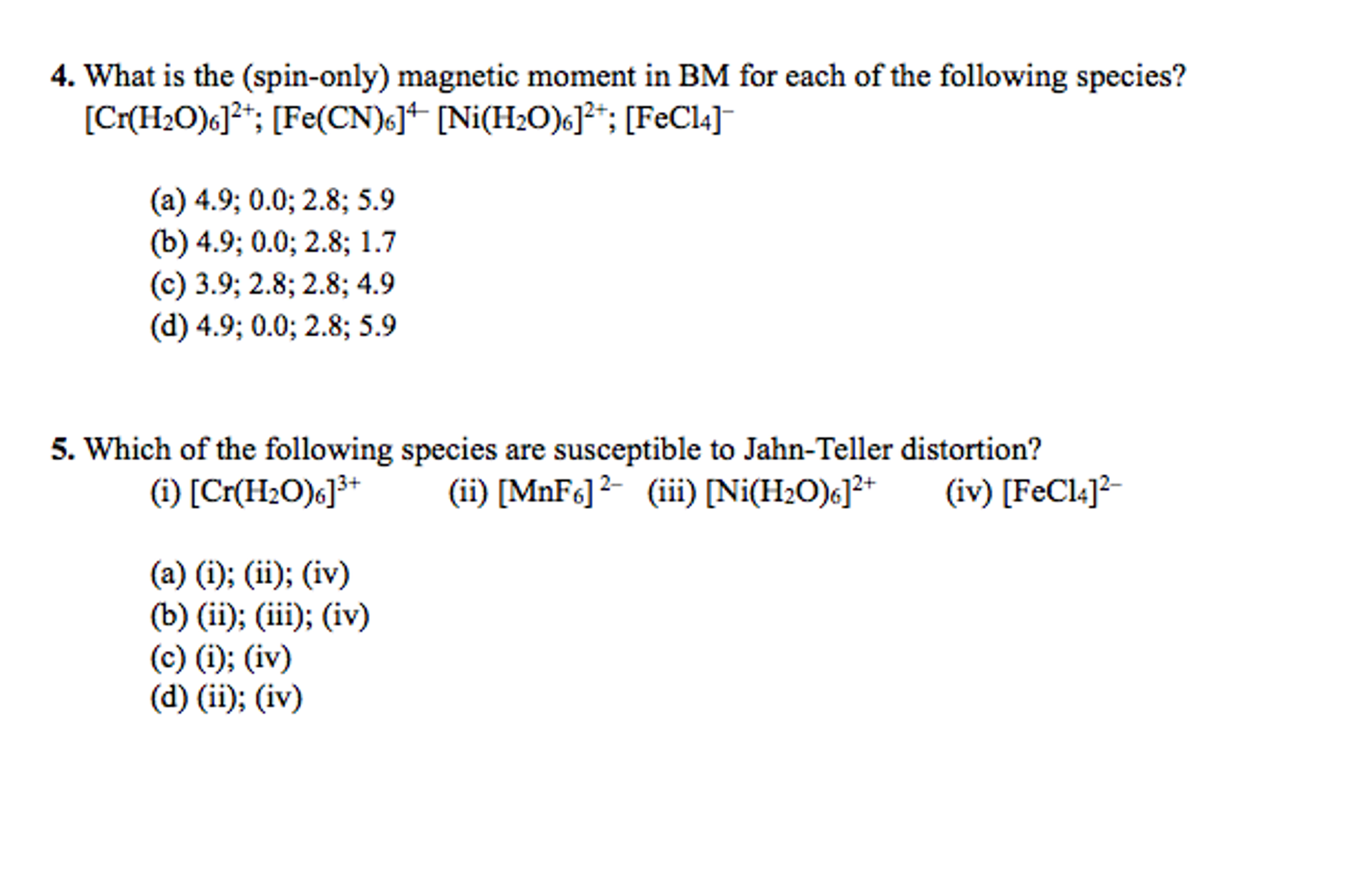
The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are
What will be the theoretical value of spin only magnetic field when Fe(SCN)3 reacts with the solution containing F ions to yield a complex

2: Average magnetic moment per Cr 3+ , Fe 3+ and Gd 3+ ion in the salts... | Download Scientific Diagram
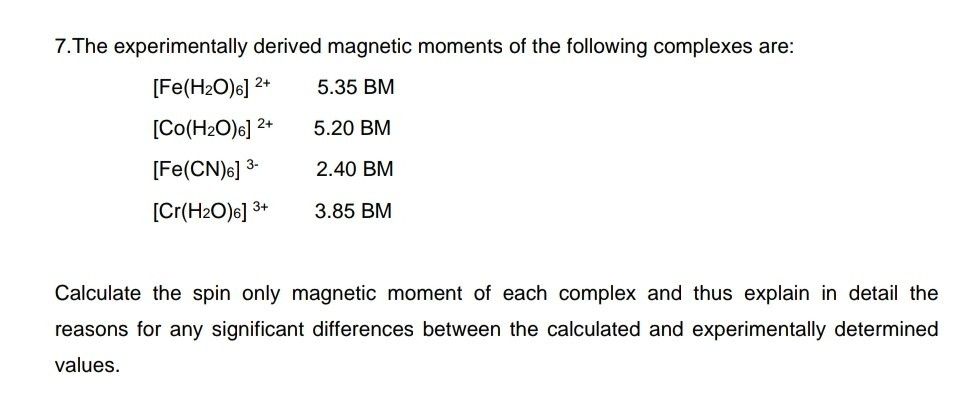
Calculate the magnetic moments of the following complexes : (i) [Fe(CN)6]-4 (ii) [FeF6]-3 - Sarthaks eConnect | Largest Online Education Community
![Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM where as [Fe(CN)(6)]^(3-) has a value of only 1.74 BM ? Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM where as [Fe(CN)(6)]^(3-) has a value of only 1.74 BM ?](https://d10lpgp6xz60nq.cloudfront.net/ss/web/584381.jpg)






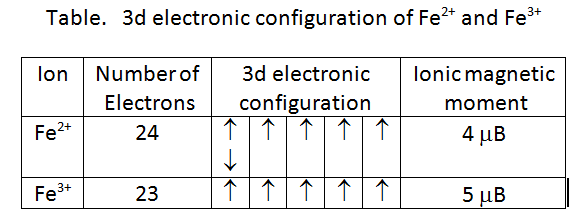


![Calculate magnetic moment of Fe^3 + in [Fe(CN)6]^3 - and in [Fe(H2O)6]^3 - . Calculate magnetic moment of Fe^3 + in [Fe(CN)6]^3 - and in [Fe(H2O)6]^3 - .](https://haygot.s3.amazonaws.com/questions/1576306_1732047_ans_78403cb9299c4e63a40902c700aaa8a9.jpg)
![The spin magnetic moment of iron in K3 [ Fe (CN)6 ] is : The spin magnetic moment of iron in K3 [ Fe (CN)6 ] is :](https://haygot.s3.amazonaws.com/questions/1952807_1117559_ans_880cbb7d24bb4d01ab49a890bc90ed14.jpg)

![The spin magnetic moment of iron in `K_(3)[Fe(CN)_(6)]` - YouTube The spin magnetic moment of iron in `K_(3)[Fe(CN)_(6)]` - YouTube](https://i.ytimg.com/vi/m2ePFFONgdA/maxresdefault.jpg)