Ph. Eur. Reference Standards: Orders and Catalogue - European Directorate for the Quality of Medicines & HealthCare

Ph. Eur. Reference Standards: Orders and Catalogue - European Directorate for the Quality of Medicines & HealthCare
2 new Ph. Eur. reference standards and 5 replacement batches released in August 2022 - European Directorate for the Quality of Medicines & HealthCare

Food-inspired innovations to improve the stability of active pharmaceutical ingredients - ScienceDirect

European Pharmacopoeia (Ph. Eur.) 11th Edition - European Directorate for the Quality of Medicines & HealthCare
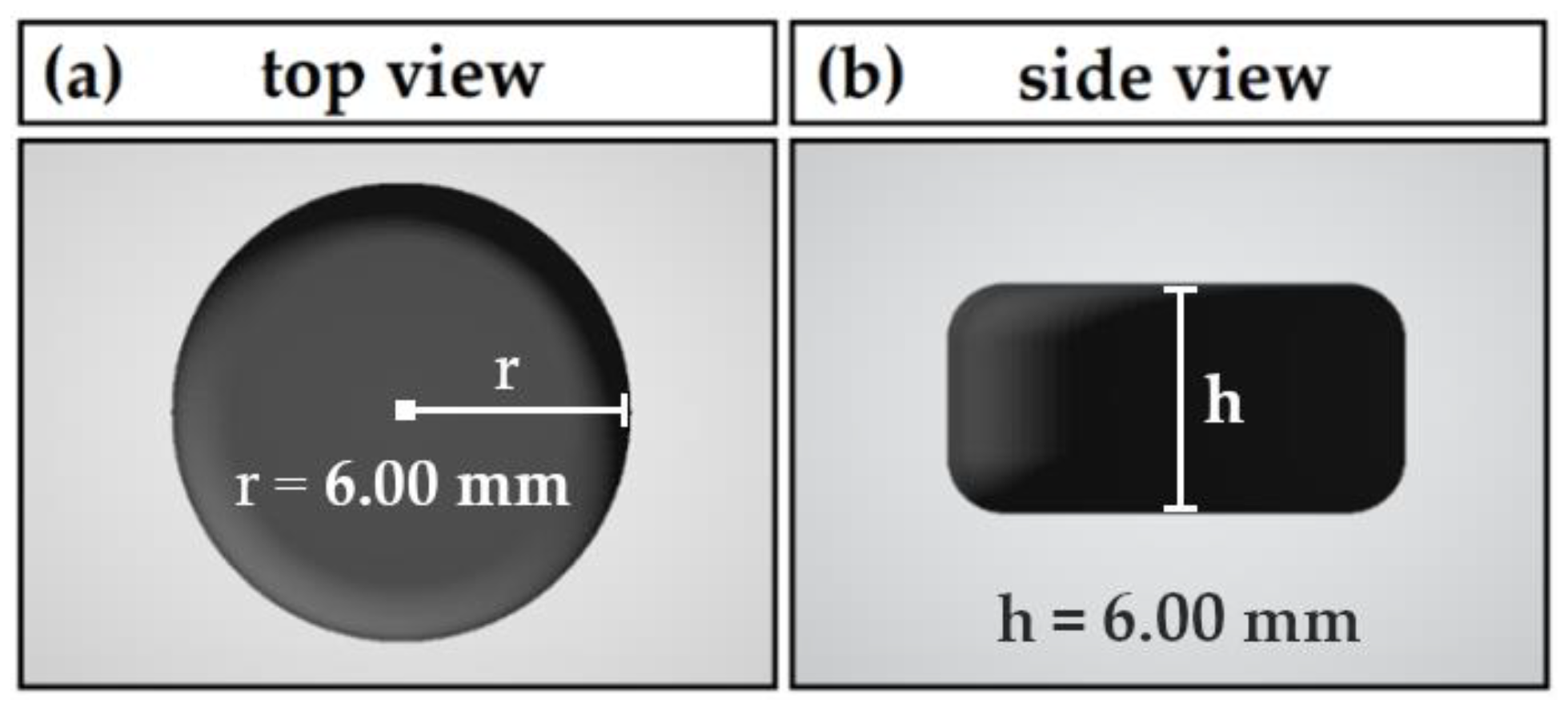
Pharmaceutics | Free Full-Text | Novel Approach to Pharmaceutical 3D-Printing Omitting the Need for Filament—Investigation of Materials, Process, and Product Characteristics
European Pharmacopoeia (Ph. Eur.) 11th Edition - European Directorate for the Quality of Medicines & HealthCare

Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration - ScienceDirect

![European Pharmacopoeia [10 ed.] 9789287189127 - DOKUMEN.PUB European Pharmacopoeia [10 ed.] 9789287189127 - DOKUMEN.PUB](https://dokumen.pub/img/european-pharmacopoeia-10nbsped-9789287189127.jpg)